|
|
|
| Single Molecule Imaging |
Stimulated Emission Microscopy
The colorful world around us as seen through our eyes is primarily due to absorption contrast – molecules absorb light of certain colors because of their intrinsic energy levels. Incident white light contains all colors. Molecules absorb some colors, allowing us to see the colors of the remaining light. An exception is the blue sky, which is due to light scattering induced by small particles and/or air density fluctuation.
Under an optical microscope, however, absorption contrast is not the method of choice for visualization for two reasons: first, absorption is too weak to measure because of the small number of molecules in a microscopic sample (the signal is much weaker than those in a conventional UV-vis spectrometer, the sensitivity limit of which is about one part per ten thousand light attenuation). Second, light scattering complicates the absorption signal as both effects result in light attenuation. For these reasons, fluorescence microscopy has been the dominant contrast mechanism for optical microscopy.
Many important molecules, such as hemoglobin, cytochrome, melanin, and retinal, absorb but have undetectable fluorescence, yet their absorption is too weak to detect under a normal microscope. In 2009, we demonstrated stimulated emission microscopy (1,2) by exploiting stimulated emission, a phenomenon that was first described by Albert Einstein and was the basis for LASER, but had not been used as a contrast mechanism for optical imaging. Stimulated emission microscopy allows orders of magnitude higher sensitivity for imaging molecules that absorb, but do not fluoresce. It allows spectroscopic identification of molecules in living organisms and is free from the complication of light scattering by the sample. The sensitivity that approaches the single molecule limit was accomplished by the modulation transfer measurement (3).
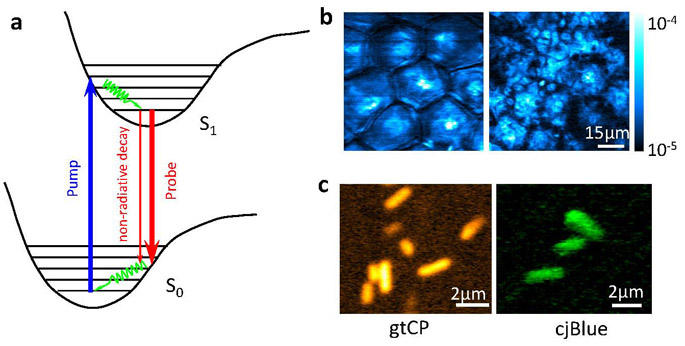
Stimulated emission microscopy. (a) Energy diagram of stimulated emission. (b) A pair of stimulated emission images of toluidine blue O, a drug used as a photosensitizer in photodynamic therapy, at two different z depths (3 and 25μm, respectively), delivered onto a mouse ear. Optical sectioning is evident. (c) Stimulated emission images of genetically encoded nonfluorescent chromoproteins, gtCP and cjBlue, inside Escherichia coli cells that contain corresponding expression plasmids.
We note that Warren Warren’s group used the modulation transfer approach to achieve nonlinear imaging based on two photon absorption (4) and excited state absorption (5) contrast. Together with these techniques, stimulated emission microscopy opens new possibilities for biomedical imaging, such as label free mapping drug distributions and blood vessels in tissues, as demonstrated by the paper.
References:
| 1. |
Min, W.; Lu, S.; Chong, S.; Roy, R.; Holtom, G. R.; Xie, X. S. Imaging Chromophores with Undetectable Fluorescence by Stimulated Emission Microscopy. Nature 2009, 461, 1105. |
| 2. |
News and Views by Hell, Stefan; Rittweger, Eva. "Light from the Dark," Nature, 461, 1069. |
| 3. |
Min, Wei; Freudiger, Christian W.; Lu, Sijia; Xie, X. Sunney "Coherent Nonlinear Optical Imaging: Beyond Fluorescence Microscopy," Annu Rev Phys Chem 62, 507-530 (2011). |
| 4. |
Ye, T.; Fu, D.; Warren W.S. “Nonlinear absorption microscopy,” Photchem. Photobiol. 85, 631-45. |
| 5. |
Fu, D.; Ye, T.; Matthews, T.E.; Yurtsever, G.; Warren, W.S. “Two-color, two-photon, and excited-state absorption microscopy” J. Biomed. Opt. 12:054004 |
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
