In all kingdoms of life, transcription factors (TFs) regulate gene expression by site specific binding to chromosomal DNA, preventing or promoting the transcription by RNA polymerase. The lac operon of Escherichia coli, a model system for understanding TF-mediated transcriptional control, has been the subject of extensive biochemical, structural, and theoretical studies since the seminal work by Jacob and Monod. However, direct observation of TF mediated gene regulation remains difficult, because TFs exist in small numbers and require single molecule sensitivity. We made the first real-time observation of specific binding of a lac repressor, labeled with a fluorescent protein, to a chromosomal lac operator. We measured the kinetics of binding and dissociation of the repressor in response to metabolic signals [1].
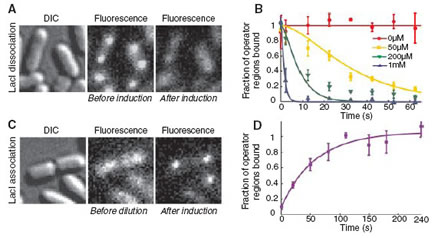
Fig. 1 Lac repressor kinetics in living E. coli cells. (A) Bacteria before and 40 s after addition of IPTG to a final concentration of 1 mM. (B) Fraction of the lac operator regions that is TF-bound is plotted as a function of time after induction by various concentrations of IPTG. (C) Bacteria before and 1 min after dilution of IPTG from 100 to 2 mM. (D) Fraction of the operator regions that is TF-bound as a function of time after rapid dilution of transcription factors from 100 to 2 mM. The data are fitted with an exponentially distributed binding time and yield a time constant of 60 s.
A central question in molecular biology is how a TF molecule or any DNA binding protein searches for specific binding sites on DNA. Target location by TFs is believed to be achieved by facilitated diffusion, in which a TF searches for specific binding sites through a combination of 1D diffusion along a short DNA segment and 3D translocation among DNA segments through cytoplasm [2]. However, direct observation in living cells has not been possible.
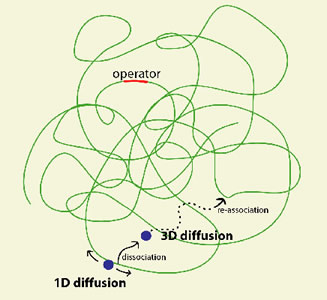
Fig. 2 Facilitated diffusion, a combined 1D and 3D search
We carried out a live cell study of the search process [1]. Specifically, we characterized the nonspecific binding to DNA, 1D diffusion along DNA segments, and 3D translocation among segments through cytoplasm. We found that in searching for the operator, a lac repressor spends ~90% of time nonspecifically bound to and diffusing along DNA with a residence time of <5 milliseconds. This gives the first quantitative measurement of how individual DNA binding proteins search for their target sequences in a living cell.
References
[1] Elf J, Li GW &Xie XS (2007) Probing transcription factor dynamics at the single-molecule level in a living cell. Science .316 :1191-4
[2] Berg, O.G.; Winter, R.B; von Hippel, P.H. “Diffusion-Driven Mechanisms of Protein Translocation on Nucleic Acids. 1. Models and Theory?” Biochemistry 20, 6929 (1981).
