|
|
|
| CARS Applications |
| |
 |
| |
Applications of CARS Microscopy
Coherent Raman microscopy (CRM) is a chemically-selective imaging modality that provides microscopic contrast without the need of labels. It is a rapidly developing technique that is constantly finding new and exciting applications. Here we show some examples of our work in the past a few years.
Studying Lipid Metabolism
Many experiments in cell biology are limited by the use of perturbative labels. Fluorophores, beads, or metal particles can have large effects on the behavior of a labeled molecule or organelle in a living organism. Lipid droplets have recently emerged as important intracellular organelles relevant for widespread human diseases such as diabetes, hepatosteatosis and atherosclerosis. The ability to visualize lipid droplets within cells and study their biogenesis and function, however, is hampered by the lack of specific staining dyes, as well as the aforementioned problems associated with labeling. CRM’s exquisite sensitivity to lipids makes it an ideal tool to study lipid cell biology.
We used RNA interference screening to study the regulatory genes affecting the lipid storage in C. elegans [1]. Figure 1 shows the SRS image of wild-type and mutant C. elegans at 2845 cm-1. The contrast is mostly from lipids, revealing the lipid content in the intestine.
|
| |
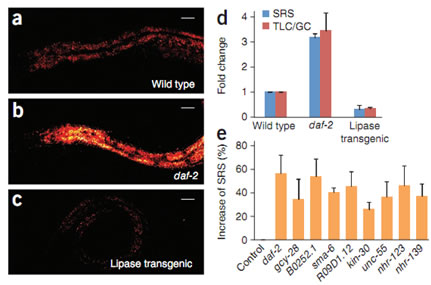
Fig. 1. RNAi screening of new fat storage regulatory genes based on in vivo lipid quantification using label-free SRS microscopy. SRS images at 2845 cm-1 for wild-type worm (a), the daf-2 mutant (b), and the transgenic worm intestinally overexpressing the lipase (c). (d) Quantification of fat content by SRS (n = 5 worms) and thin-layer chromatography–gas chromatography. (e) SRS signal increase compared to the control for genes that resulted in a fat content increase of more than 25% when inactivated by RNAi (P< 0.0001, n = 5 worms).
|
| |
Imaging Drug Delivery
Coherent Raman microscopy can be used to study drug-cell and drug-drug interaction at subcellular resolution in living cells. In order to distinguish drug from cellular components, hyperspectral SRS data is required. We took hyperspectral SRS images of imatinib (trade name Gleevec) and nilotinib (trade name Tasigna) treated mouse leukemia cells and found that these drugs have a surprisingly large enrichment inside lysosomes. The enrichment is due to the lysosomotropic properties of these drugs: weakly basic drugs are protonated in lysosomes (thus are charged) and then trapped inside. However, this effect can be reversed by using a secondary drug chloroquine, which interacts with Gleevec through acid-base equilibrium in the lysosome and drives out Gleevec (thus increases its efficacy). For Tasigna, the same approach does not work at high Tasigna concentration because it precipitates inside lysosome before the pH of the lysosomes can be changed by chloroquine.
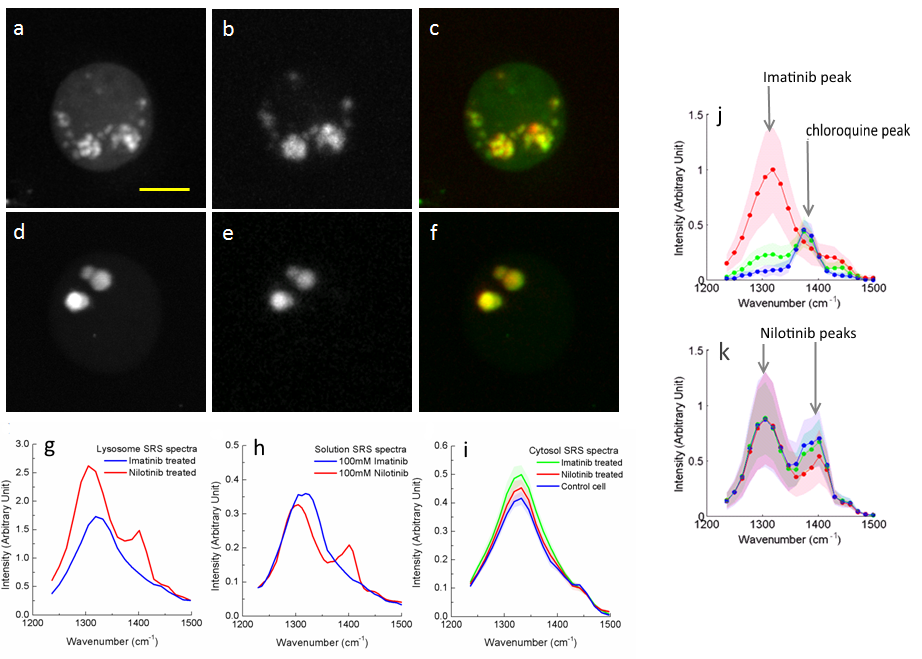
Fig. 2.Imaging drug uptake and distribution inside living cells. Leukemia cells are treated with 20 μM imatinib or nilotinib for 2 hrs. (a-c) SRS and two-photon fluorescent image of imatinib treated cells: a. SRS image at 1305 cm-1; b. fluorescent image from lysotracker staining; c. color overlay of the two images. (d-f) SRS and two-photon fluorescent image of nilotinib treated cells: d. SRS image at 1305 cm-1; e. fluorescent image from lysotracker staining; f. color overlay of the two images. (g). SRS spectra of drugs accumulated in lysosomes. (h) SRS spectra of drugs in solution. (i) SRS spectra of the cytosol. (j). Lysosomal SRS spectra of imatinib and chloroquine treated cells: red curve: 20μM imatinib; green curve: 20μM imatinib, 20μM chloroquine; blue curve: 20μM imatinib, 50μM chloroquine. (k). Lysosomal SRS spectra of imatinib and chloroquine treated cells: red curve: 20μM nilotinib; green curve: 20μM nilotinib, 20μM chloroquine; blue curve: 20μM nilotinib, 50μM chloroquine.
Tumor Diagnosis
CRM microscopy provides an interesting new contrast mechanism for imaging tumors in tissue. In particular, by making use of differences in lipid density and protein density, the nuclei morphology can be derived, providing contrast analogous to H&E histopathology. Tumor margins can be seen with subcellular spatial resolution in fresh, unstained tissue.
Figure 3 shows the post-processed SRS images of both primary and metastatic tumors, highlighting the lipids (green) and nucleic contrast (blue). As can be seen, not only the tumor margin can be identified, but the margin of the primary tumor and metastatic tumor are also distinguishable. Comparison with histological data and Raman spectroscopy has established the chemical selectivity of the technique, and efforts to establish the use of CRM as a tool for virtual histology during brain tumor surgery are underway.
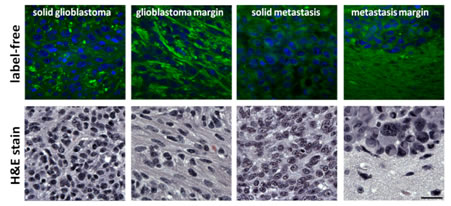
Fig. 3. SRS images of glioblastoma multiform (primary brain tumor, left) and breast cancer brain metastasis (secondary tumor, right) reveal tumor margin.
It is also possible to obtain whole mouse brain imaging by stitching many SRS images together. Figure 4 shows the image of a mouse brain section about 2 mm in thickness. Anatomy of the brain can be clearly delineated with the two color image.
Fig. 4. SRS image of sectioned healthy mouse brain.
Applications outside Biomedicine
As a chemically sensitive technique, CARS microscopy has many applications outside biomedicine. One example is studying degradation of cellulose and lignin for biofuel production. Research of biofuels has experienced dramatic growth in recent years. The major challenge to be overcome in the widespread adoption of many biofuels is that biomass is intrinsically recalcitrant, partly caused by one of the two main chemical species in biomass, lignin. The SRS microscopy method can offer new information on the biomass conversion processes. Figure 5 shows the real-time monitoring of the hydrolysis kinetics of cellulose and lignins under chlorite treatment.
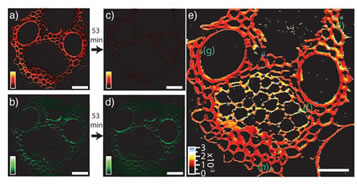
Fig. 5. Real-time SRS imaging of a delignification reaction in corn stover. a) and c) Lignin signal at 1600 cm-1 before and 53 min after chlorite treatment reaction. b) and d) Corresponding cellulose signal at 1100 cm-1. e) color-coded rate constant of lignin degradation.
Imaging Neurotransmitter Acetylcholine
Acetylcholine (ACh) is an important neurotransmitter that relays neural excitation from lower motor neurons to muscles in a structure called neuromuscular junction (NMJ). It also plays significant roles in the central nervous system by modulating neurotransmission. However, there is a lack of tools to directly measure the quantity and distribution of ACh at the subcellular level. Even though ACh is highly concentrated at NMJ ~ 30 ml on average, it’s still on the lower detection limit of SRS, the background from the nearby tissues also make it difficult to detect ACh with SRS.
We used frequency-modulated spectral-focusing stimulated Raman scattering (FMSF-SRS) to remove imaging backgrounds. Moreover, with spectral analysis, we estimated the local concentration of acetylcholine to be about 7 - 13 mM at the neuromuscular junction of frog cutaneous pectoris muscle.
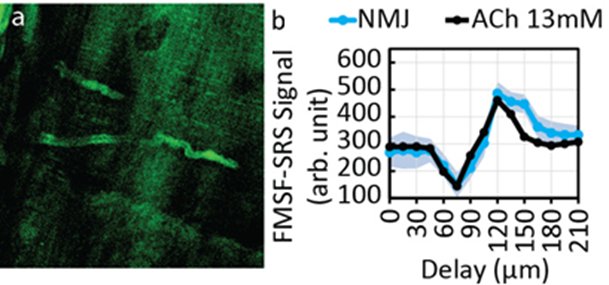
Fig. 6. a) FMSF-SRS image of a frog NMJ. The NMJ has high signal level. b) Spectral scanning of frog NMJ with FMSF-SRS to confirm and measure the concentration of ACh at frog NMJ.
Monitoring ALS in Mouse Model
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease. 50% of ALS patients die within 30 months within symptom. There is no definitive diagnosis for ALS and there is no cure for ALS. Improved methods for monitoring motor neuron degeneration are much needed. SRS microscopy of lipid is used for visualizing the myelin at the sciatic nerve of a mouse model of ALS SOD1G93A. The lipid rich ovoids in the sciatic nerve of SOD1G93A mice is found to be a reliable biomarker of the disease. It is shown that we can distinguish diseased mice and healthy mice as early as 5 weeks by processing the SRS images with a newly developed quantification algorithm. SRS microscopy and the quantification method is shown to be one of the earliest biomarkers for ALS in SOD1G93A mice. It is shown that this method can be used in drug testing on mice and similar lipid ovoids are also visible in motor nerves of human ALS patients (postmortem).
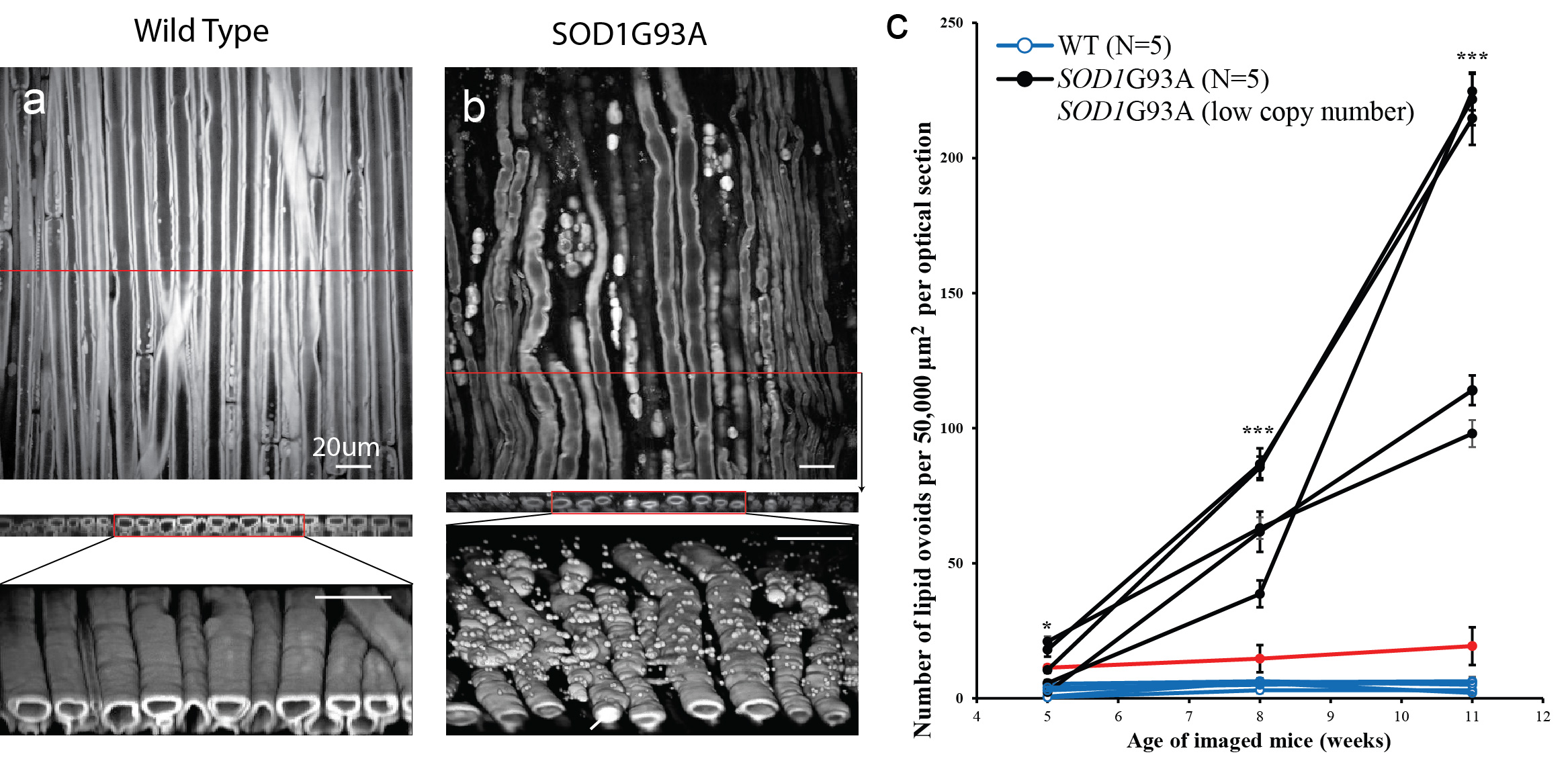
Fig. 7. Three-dimensional SRS imaging, cross section and reconstructions of sciatic nerves of a wild type mouse aa and SOD1G93A mouse b), at 2850 cm-1. c) Tracking of the number of lipid ovoids of SOD1G93A and wild type mice.
|
| |
References:
[1] Wang, Meng C.; Min, Wei; Freudiger, Christian W.; Ruvkun, Gary; Xie, X. Sunney "RNAi screening for fat regulatory genes with SRS microscopy" Nat Methods 8, 135-138 (2011).
[2] Fu, Dan; Zhou, Jing; Zhu, Wenjing Suzanne; Manley, Paul W.; Wang, Y. Karen; Hood, Tami; Wylie, Andrew; Xie, X. Sunney. "Imaging the Intracellular Distribution of Tyrosine Kinase Inhibitors in Living Cells with Quantitative Hyperspectral Stimulated Raman Scattering, Nat Chem 6, 614-622 (2014)
[3] Saar, Brian G.; Zeng, Yining; Freudiger, Christian W.; Liu, Yu-San; Himmel, Michael E.; Xie, X. Sunney; Ding, Shi-You "Label-Free, Real-Time Monitoring of Biomass Processing with Stimulated Raman Scattering Microscopy" Angew. Chem. Int. Ed. 49, 5476-5479 (2010).
[4] Fu, Dan; Yang, Wenlong; Xie, X. Sunney "Label-free Imaging of Neurotransmitter Acetylcholine at Neuromuscular Junctions with Stimulated Raman Scattering," J Am Chem Soc 139(2), 583−586. DOI:10.1021/jacs.6b10727 (2017)
[5] Tian, Feng; Yang Wenlong; Mordes, Daniel A; Wang, Jin-Yuan; Salameh, Johnny S; Mok, Joanie; Chew, Jeannie; Sharma, Aarti; Leno-Duran, Ester; Suzuki-Uematsu, Satomi; Suzuki, Naoki; Han, Steve S; Lu, Fa-Ke; Ji, Minbiao; Zhang, Rosanna; Liu, Yue; Strominger, Jack; Shneider, Neil A; Petrucelli, Leonard; Xie, X Sunney; Eggan, Kevin "Monitoring peripheral nerve degeneration in ALS by label-free stimulated Raman scattering imaging," Nat Commun 7:13283. DOI: 10.1038/ncomms13283 (2016)
|
|
|

